Christians are so cringe.
I’d rather hang out with the crystal people.
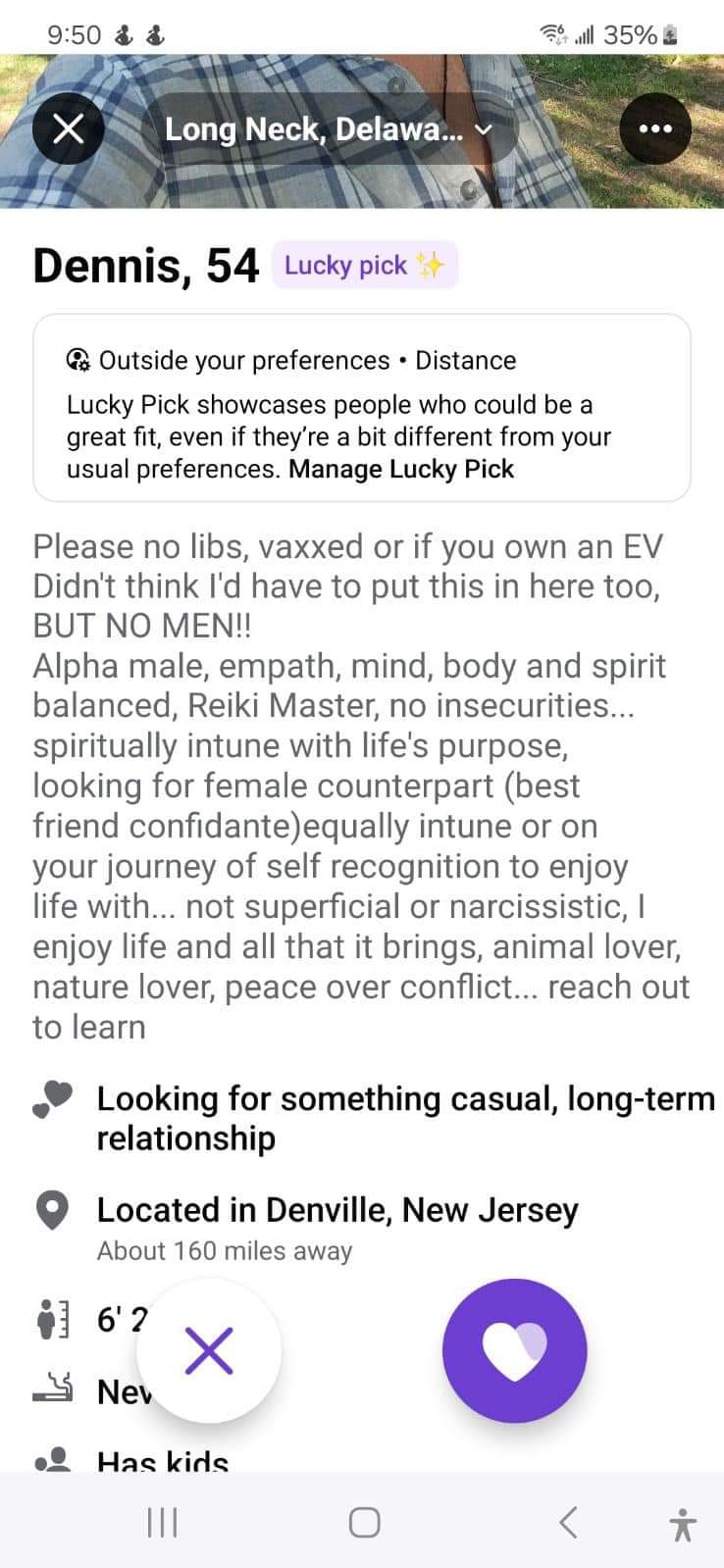
Idk some of them are this bad.
> Alpha male
> no insecurities
Hmm.How is someone into Reiki this big of an asshole? I’m not saying I am into Reiki, but it sure doesn’t jive with the rest of him.
Not narcissistic
I mean it’s hard to be more narcissistic than this
“Alpha Male”, “empath” and “no insecurities” in the same fucking sentence is some TRUMP level lying 😄
Ooooo lucky pick.
Funny, because he looks like a basic bitch
Nature lover
No EV
Guess he wants a cyclist girl?
Alpha male? Is it a furry thing?
I think it’s a toxic masculinity thing.
I’ve at least seen a crystal. Some of them even actually do things.
I try to hang out with them but my eyes keep rolling out of my sockets
Oxygen is measurable. We can detect even tiny amounts of it, we know its makeup, we have well characterized its behavior, and we can make it work for us.
We have no evidence for the existence of any gods. Seems like we can exist without them just fine.
Bro, you can literally look at pictures of Vissarion. God lives in Siberia. 🙄
You almost had me, but his Wikipedia lists his profession as “spiritual teacher,” not “God.”
That was written by a heretic.
He’s Christ bruh, just look at him.
wb the Quran? The only book uncorrupted in its existence. In it God says He will preserve the book, and if it is a fabrication He challenges you to produce even a single Suraht (chapter) like it. Also neither God nor the angels will appear until the Day of Judgement, so asking for either while you persist in disbelief is kind of a bad idea.
Bring on the downvotes, it’s the worst/best you can do.
Look at you, bringing this thread back from the dead.
First of all, even if we take what you’re saying at face value, how does it being an original text in any way prove that its contents are true? If I made up a completely original story today about a stuffed animal that eats pickles and poops diamonds, would that mean that such a thing exists?
Secondly, we can’t take what you’re said at face value because Qur’anic and Muslim scholars are very divided about the origins of the Quran.
So you’re saying we just need to freeze god to see him?
It’s worth a try. We need to get some revenge and revenge is a dish best served cold.
I used to think that saying meant that revenge was ice cream when I was a kid. Mmm… Ice cream… 🤤
I guess you hadn’t seen Star Trek II…

And where’s God? Up in the sky. In space.
Mmm, tasty god
Yes, it’s the cold truth
God is just on the other side of absolute zero kelvin, right over there.
We need to liquefy God
And make him into the most artisinal smoothie in all of Portland!

Ask and you shall receive - Toast
This looks like someone who has used a soldering iron to draw Jesus on a piece of toast and then countersunk it into a second larger piece of toast.
Which I doubt was easy.
The “image” is on a piece of cheese.
Cheesus Crust
, on toast.
That’s Costa

Holy mother Mary and Joseph! Do my eyes deceive me or is that the son of God etched into my sandwich!? Could it have been divine intervention that compelled me to put the cheese on the outside?! Literally no other explanation exists for why I might’ve done that!
That reminds me of a picture of “Jesus” that kept bleeding through the paint in one of my old landlord’s apartments. He tasked me with stripping the paint off the wall to find out what was actually below the white paint he had slapped up. Several layers of white paint below the surface, I found an absolutely gorgeous oil paint mural of Bob Marley.
God is like oxygen—highly volatile.
How does oxygen change on it’s own?
Well, we call it chemistry, but the forces at play existed long before we even knew to name them.
I understand. But if it’s isolated it’s considered non reactive. (I manage a hazmat DOT fleet)
Ok? That’s neat, I just fail to see the relevance I guess. A god would be harmless too, if it was isolated from the rest of the universe.
I’m asking how it’s going to react with stuff. In like a basic chemistry kind of way.
Seems like something a hazmat DOT fleet manager would already know though, so I guess I just don’t know why you’re asking that. And truthfully, I don’t particularly care, as, again, I fail to see the relevance. I mean like, if it’s real chemical kinetic information that you’re after, there are innumerable better sources than the comments section of a meme page on a fringe social media platform. If you’re trying to make a point about my original tongue-in-cheek comparison, feel free to make it.
Ok. You can just accept you’re wrong rather than double downing and sounding less educated than you originally did.
Freeze it.
Can’t say I’ve ever seen liquid oxygen.
It’s amazing. Especially when you’re trying to chill and prime a pump, and there’s gallons of it flowing across it’s own vapor in puddles.
Just try not and think about what happens if it flows across that oil spill and you step in it.
Why? What happens? Legit too lazy to try and strong arm Google into trying to tell me…
Oxygen loves hydrogen. Hydrocarbons have lots of hydrogen. Oil is hydrocarbon.
Add enough energy to start the chemical reaction, and BOOM. Along with all the things that go with exothermic and expansion reactions (ie, your foot gets blown off, and anything that can burn in the area likely will be)
Also, get things hot enough with oxygen around, and things you think can’t burn, will. Including steel.
Look up oxygen compressor fires. It’s scarily interesting.
That’s…fun…and a bit spooky. Oxygen compressor fires…yeah, goddamn wow.
Liquid oxygen is way too pretty for how dangerous it is
Ok, I’ll bite. Why/how is liquid oxygen dangerous? Doesn’t it just basically instantly boil into gas if it gets anywhere outside of its cryo chamber?
Not really, it stays liquid until it boils, so it can stay outside the cryo chamber for a few seconds. And since it’s pure oxygen, on top of the dangers with liquid gases being super cold, it’s also a potent oxidizer, so it can set fire to some fuels without a hear source
So… Mix with Florine for best results?
I realize I’m posting a complaint in my own thread so it’s my own fault, but I keep getting “Love is Like Oxygen” by Sweet stuck in my head since I’ve posted this.
Removed by mod
It’s oxygen not an entheogen
You don’t even need oxygen to be in a liquid or solid state to see it: oxygen is the reason the sky is blue. When you look through a large enough volume of gaseous oxygen, as you do when you step outside in the day time, you can see it just fine.
You could say it’s… triune!
Oxygen is absolutely measurable, and your own body will quickly tell you something is very, very wrong if your 02 intake is too low, or even weird (which is why SCUBA mixes are a topic of their own.)
There is the matter that oxygen does affect light, which allows us to tell if an exoplanet has oxygen in the atmosphere, which means it’s not invisible, just very transparent. But when we look at other worlds we detect oxygen by analyzing the light that comes from them, so we see oxygen.
In the meantime, the human species as we know it (homo-sapiens) has been around for 250,000 years. The monotheistic version has been around for about 4,000 years (and even Adonai had others in His pantheon. The temple priests of Adonai murdered Asherah, His consort, by massacring all the Asheran desciples and burning Her temples down). For most of that time, ancestors, elemental spirits and animal spirits were central to our religious faith, not high-concept deities. For the vast majority of human existence, God did not exist as He is commonly regarded in popular religions.
AFAIK, we do not, in fact, have any biological system that detects oxygen in the air. What we use instead is detection of the things that are typically present when oxygen is not. Like CO2 concentration. This is what makes a room “feel stuffy”, CO2.
I don’t think this invalidates your point at all, of course you have a valid argument despite the biological misunderstanding here.
The only reason I know this is when looking into the whole, capital punishment by nitrogen hypoxia thing, I kinda stumbled into a lot of information. We don’t generally detect nitrogen nor oxygen in any way, shape, or form, since it’s quite plentiful in the atmosphere of the earth (78% nitrogen, 21% oxygen), there’s not much reason to. We’ve never needed a biological trigger to say “there’s oxygen here” because there’s never been situations where that hasn’t really been true until very very recently (eg. Closed systems like submarines, aeroplanes, vessels that go into high orbit/space, etc).
Looking at the evolution of it, any such space will accrue toxic/deadly levels of atmospheric gases, long before the oxygen is consumed. So we have biological processes to detect atmospheric toxin levels, with one example being CO2. According to some data I’ve read, CO2 freely is absorbed and expelled by the body through the blood via the alveoli (lungs), which makes the amount of CO2 in your blood a function of atmospheric CO2 levels, which may slightly waiver due to your physical workload. As you produce CO2 within your body from metabolic activity, either from regular metabolic tasks or through physical exertion, the rise in blood CO2 levels is expelled by the blood through equilibrium with the surrounding atmosphere. Simply, if you have higher CO2 concentration in your blood than there is in the atmosphere, it will diffuse towards the atmosphere (I’ll reiterate that the process works in reverse too).
High CO2 concentration in your blood affects your blood pH and can create an acidic environment, which the body can easily detect.
As far as I’m aware, there’s no similar biological process to detect oxygen levels, either directly or indirectly.
This is the danger of nitrogen hypoxia. If you’re in a low CO2 environment which is devoid of oxygen (or has very little atmospheric oxygen, not enough to sustain human life), with most of the o2 concentration being replaced by nitrogen instead, your body can still expel CO2, but cannot obtain the o2 required to survive. Since there’s no mechanism to detect this, your blood o2 levels drop to levels which are incompatible with living while you remain unaware that a problem exists.
Thus, you can easily perish when your o2 saturation drops to nil, with no indication that you’re at risk of dying.
Sorry for the dissertation, this is just something I find incredibly fascinating about biology. I hope I didn’t bore anyone too much.
Disclaimer: I’m not a biologist or scientist, I’m just some guy with ADHD, and I’ve hyperfocused on this subject a couple of times. If anything I’ve said is incorrect, I invite corrections. If possible, please link additional resources for further reading and my ADHD brain will thank you very kindly for the effort.
happycakeday. thx for the thorough insight.
so what happens with asphyxiation by noble gases?
with my limited knowledge helium makes your voice funny and nitrogen will boil your blood.(neither of which are noble gases).A significant over infusion of nitrogen in high pressure will vaporize (forming bubbles) when pressure is suddenly decreased. Nitrogen intake at relatively the same atmospheric pressure won’t cause your blood to boil.
When scuba divers dive fairly deep, the extra pressure from the water above them is often significantly higher than atmosphere, as a result of the pressure and other factors (I’m glossing over a lot of science here), increases nitrogen content in your blood. If the pressure on your body is suddenly decreased to atmospheric pressure, then nitrogen bubbles form and it can be fatal. Most commonly it just gives you, what is referred to as, “the bends”
Simply being exposed to a high nitrogen atmosphere, without the added pressure and conditions found in scuba, your blood will not form bubbles, nor boil.
I haven’t done any examination of biological reactions to other, less common gases. Noble gases, as far as I’m aware, don’t often appear naturally, and it’s uncommon for such an environment to exist accidentally. Unless someone is storing large quantities of Noble gases in a poorly ventilated, enclosed space, while the containment vessel is leaking, I don’t think it would be possible to have such a scenario occur.
Due to this, I have never felt the need to look into it. It just seems so unlikely that anyone would find themselves in that situation, and if such a situation was likely due to workplace related hazards, special training would be provided. Got the general population, unless they happened to be in a valley where a tanker truck was transporting a heavier-than-air Noble gas, on a particularly still day, the probability of exposure is astronomical.
However, CO2 poisoning can happen in your own home. Along with CO poisoning, and more. You can literally suffocate in your own home. While fairly unlikely, it’s significantly more probable than pretty much anything else we’ve discussed. Nitrogen bubbles in the blood is only a risk for those exposed to high pressure areas, such as scuba divers, so I’d give that a second most likely, out of what we have discussed, and it’s basically impossible to end up with nitrogen hypoxia without being in very specific conditions, so that would be near the bottom of my list of what’s likely.
That being said, personally, I have plenty of CO and CO2 detectors around my living areas, and anytime there’s a rise in concentration of anything that could be harmful, I tend to take action. I also have a radon meter in my basement, which I also keep an eye on.
I already know that my home needs better ventilation to outside, and I’m considering my options for an ERV. I also need something to mitigate radon, which is an entirely other matter. Radon issues are more systemic, and less acute, so it’s a bit far down on the priority list for now. I have more urgent matters. My radon levels are not great, but not critical.
I’m a data nerd, and I’ve been tracking my living space’s atmospheric metrics for years.
wtf is Radon? never really heard of it or its potential hazards.
A radioactive gas which is naturally occurring. There’s radioactive material pretty much everywhere underground. Some places more than others, but it’s basically everywhere. These are natural radioactive deposits, and they’re usually not concentrated enough for mining or anything, it’s basically trace amounts, more or less. It’s usually deep enough that there’s no radiation making it to the surface and no danger, however, when it decays it releases radon gas, which still isn’t really dangerous unless it reaches high concentration. Since it’s a gas, it leaks up to the surface and if you have a basement especially, it can seep through into the basement, and if your home is fairly well sealed, it will accumulate.
If enough accumulates, it increases your risk of certain types of cancer.
I’ll be clear, the danger of radon is in long term exposure to relatively “high” concentrations of the gas. If you monitor it and take measures to reduce the levels actively and on an ongoing basis, you’re probably fine, since the levels are, on average, below the danger zone. If you’re consistently above danger levels, then it’s possible that you could get cancer as a result.
If you don’t have a basement, you’re basically immune to having a concern. Unless your ground floor is on a concrete slab on the ground or something, which might pose a risk of it seeping in.
Radon has a half-life of a few weeks, so to reach the accumulation and duration requirements for danger, it would need to be a fairly consistent flow into the home, with little to no ability for it to escape into the atmosphere outside.
There’s a few ways to mitigate it, including radon vents under the home, or a ventilation system to exchange the homes air volume outside, something like a whole home ERV may be enough to remove/reduce it.
Highest risk would be those in older homes which have a basement, with more modern “high efficiency” insulation techniques, which generally cause less leakage of inside air to outside air (and vice versa), which are almost consistently sealed with heat/AC on.
I’m at risk since I believe this home is between 20-40 years old, so it was built on or before the 2000’s, when they had pretty good home insulation, but didn’t have the option of an energy recovery ventilator at the time. The windows have been upgraded which sealed the house further. We have a reasonably sized basement, and no ERV at this time. I’m planning on adding a few ERV units and a radon ventilation system (either under-home or in the basement) to vent it safely away from the house. The ERV units are mainly for CO2, but they should help with radon as well, and having a dedicated radon vent system in the basement should drop levels well into the safe zone.
If your house has a crawlspace underneath instead of a basement, one which is not in the insulated envelope of the home, then there’s no risk, since the crawlspace is where radon would have an opportunity to accumulate, but it’s vented directly with the outside atmosphere, giving the gas an unrestricted path away from your living space. Anything built above ground level, such as condos or apartments have basically zero risk, unless they share ventilation with an underground area, which most do not.
I picked up an airthings radon detector and levels fluctuate wildly for us. From 46 Bq/m3 to a little less than 600 Bq/m3. “Safe” levels are below approximately 100 Bq/m3, and “danger” levels are above 150 Bq/m3. Between those is the “warning” levels. My meter is in the basement, not my living areas, so it’s showing the worst possible numbers, which may not be representative of levels in living/sleeping areas, since the basement has no HVAC vents/returns, and would be fairly stagnant. The basement is also the last place in my home to be ventilated, since we tend to open windows in living areas, leaving any windows in the basement closed. So I expect the numbers in the rest of my home to be less than whatever the meter is showing at any given time. AFAIK, radon is generally heavier than air as well (though, not by a lot), so it should also settle in the basement, driving the reading up even further.
Since it takes years of danger level exposure to increase the likelihood of cancer, and other factors like CO2 can concentrate to toxic levels in a matter of days, even in a fairly large, but well sealed home, I’m more focused on CO2. Our CO2 levels when the house is sealed, can go above 2000 ppm, which is normally when I feel like I need to take action, usually it takes about 30hrs or so to go from atmospheric CO2 levels (when the home is open, around/below 450ppm) to 1800+ but it can happen more quickly if we have more people in the home. Typically we have four adults, and five pets. I imagine we would hit toxic levels of CO2 within a week of having the home sealed with typical occupancy (around 4000-5000 ppm is when it gets dangerous).
I’m acutely aware of my dangers, and I’m currently trying to save money to deal with both issues permanently.
wow. well TIL. the more you know… thx for the insight
Iirc it’s the main reason (or one of them) that non smokers get lung cancer.
I would guess asbestos etc to be the culprit.
But how do smokers of 30+ yrs never get lung cancer? the world works in mysterious ways.Asbestos is no good for sure, so in was curious to go check. The cancer.org folks do say radon is the second cause of lung cancer overall, and the #1 cause for non smokers.

How cold do we gotta get God in order to see him?
Colder than absolute zero.
God is a gas because he’s not cool enough















