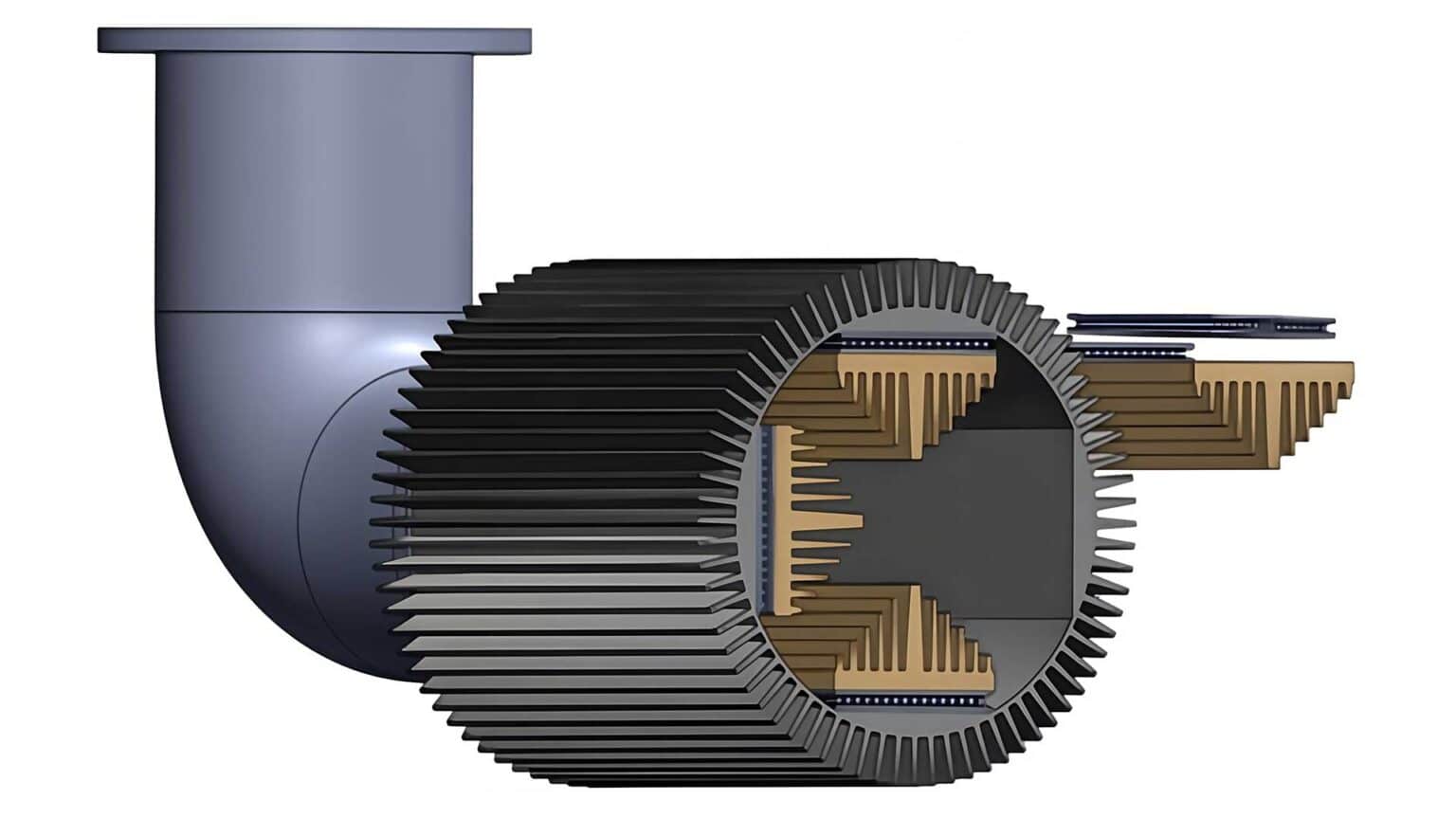
This device attaches to a car’s tailpipe, capturing heat and converting it into usable electricity. The researchers’ innovative system includes a semiconductor made of bismuth-telluride and uses heat exchangers—similar to those found in air conditioners—to capture heat from vehicle exhaust pipelines efficiently.
Basically, slap Peltier modules on the exhaust pipe. This ain’t gonna do much. We can invent a thousand applications for Peltier modules, until there is a massive technology breakthrough in terms of semi-condutor materials, it’s kinda pointless.
Reusing heat energy from exhaust is what turbos are doing for 120 years now.
I thought turbos converted air (exhaust) kinetic energy into mechanical energy, not the heat itself. If exhaust was cold, the turbo would still work, no?
Not saying that these Peltier devices are going to be game changers, but it sounds like they do something different from turbos in that they are capturing additional wasted energy.
Yes, and no. Heat and kinetic energy are fundamentally all just energy. What we call heat is, technically, the kinetic energy of molecules vibrating around.
When exhaust gas passes through a turbocharger, it is both slowed and reduced in pressure, resulting in it coming out slightly cooler than when it entered. This device is using a different method of getting energy out of the exhaust gas, but it’s fundamentally still the kinetic energy of those very energetic exhaust gas molecules bouncing against one side of the thermoelectric generator and giving up their energy into it. I would still expect the exhaust gas to come out of it slightly cooler and slower.
Your explanation about where the energy comes from with turbochargers sounds wrong to me.
When exhaust gas passes through a turbocharger,
You’re skipping a crucial step here. The exhaust gases get pushed through input of the exhaust gas impeller on the turbocharger by the movement of a piston in the engine during the exhaust cycle. This “work” isn’t free. Its energy that comes from the other pistons on their combustion cycle. If there is more resistance on the exhaust coming out of the engine (which there is to drive the turbocharger impeller), that energy must be added (robbed) by the energy at the crankshaft that ultimately powers the wheels.
The extra boost of power we experience in an engine from using a turbocharger is that the turbocharger allows more oxygen to be put into the combustion chambers (and the engine puts more fuel in at the same time). The extra energy is from burning - - more fuel in the same period of time than without turbocharging. The fuel is the source of the energy, the turbocharger isn’t recovering any energy.
The article is covering technology is actually recovering energy turning heat (thermal energy) back into electricity (electrical energy).
The exhaust gases are at a high pressure after combustion due to combustion heat. The turbo does indeed increase exhaust pressure, and therefore extracts some work from the crank but it’s extracting significantly more from the high pressure of the expanded hot gas. It’s not “free” because it’s energy that is usually just wasted in a naturally aspirated engine. There are many examples of engine configurations where a turbo is used to boost efficiency by reducing displacement.
There were systems on old aircraft engines which used exhaust power recovery turbines geared directly to the crank. Those wouldn’t physically function under your concept.
The increase in manifold pressure doesn’t just increase oxygen in the cylinder. It also increases the manifold pressure, or the total mass of gases. The increase of oxygen does allow for more fuel and total energy in the ignition event but the extra
inertgas also expands when heated. So both play a factor in increasing mean effective pressure, and therefore energy output per cycle (power).Edit: im tired… Bad wording, adding inert gas to increase intake mass doesn’t help.
The turbo does indeed increase exhaust pressure, and therefore extracts some work from the crank but it’s extracting significantly more from the high pressure of the expanded hot gas.
I’ll admit I’m at the edge of my knowledge here, but are you saying that if we were increasing the pressure in the cylinder from, say pure nitrogen (or another inert), instead of atmosphere (which contains oxygen), and we kept the same amount of fuel from natural aspiration, we’re still get the majority of the benefit of turbocharging even overcoming the parasitic portion of extra energy needed during the compression cycle and the exhaust cycle against the turbocharger impeller?
No. The heat of combustion increases the gas temperature. But this temperature increase is relative to the mass of the gas. The heat is relative to fuel/oxygen mass combusted. (Combustion energy + Ideal gas law)
Add mass without adding combustion, you get lower pressure and temperature out. So you get less boost from the turbo and make more work for the compression cycle.
The major point of the turbo is to use wasted heat to add more oxygen by packing more air in. So it’s a bit of an odd question to answer. The point is there’s a lot of energy wasted in a naturally aspirated engine’s exhaust. Turbos mostly use that wasted energy, and not power from the crank.
Oh yeah, the turbo is going to have an efficiency ratio for converting exhaust pressure into boost. So that added backpressure on the exhaust is going to be offset in the intake stroke by that ratio. Not important to the point, hat a tidbit. These things are so complicated lol.
Gotcha, thanks. It doesn’t sound particularly revolutionary as far as energy capture goes, since 40W is probably negligible on today’s cars.
NGL though, on an old '75 Honda motorcycle I used to own, an extra 40W would have been amazing. The alternator was too tiny to keep the electronics running and battery charged, and I had to turn the headlight off whenever possible to keep the battery charging.
What we call heat is, technically, the kinetic energy of molecules vibrating around
I’ve wondered about this. If this is so, and heat is molecules moving back and forth, how do the molecules stop, change direction, and then accelerate in the other direction, stop, change directions again, and go back, over and over and over?
Yeah, I do apologize - I’m somewhat simplifying my explanation because when you start going into the full detail, it just brings up more questions.
So yes, like the other comment says, the particles are constantly bouncing into other things.
- If they’re bounded in by something - walls of a container, or even just more gas surrounding the specific sample you’re looking at - they’ll bump into that, and transfer some of their energy to that.
- If they don’t have something to bump off of and the particles are free-floating, they’ll take off in any given direction. If they only have something to bump off of in a limited number of directions, they’ll take off in the other direction. (For instance, in a rocket engine, we make a lot of molecules really, really hot and then surround them with barriers in every direction except the one we want them to zoom out in.)
- In some cases, the molecules have electromagnetic bonds with each other, which take more energy to break than the energy contained in their “bouncing around”. So they’ll stay stuck, just bouncing off each other, even in a vacuum, (Or at least, until they radiate away their heat via electromagnetic energy… another whole story.)
Nothing to apologize for - thank you for elaborating.
deleted by creator
It’s pretty much like billiards. They just bounce. Different chemicals (types of molecule) are different phases at different temperatures e.g. nitrogen is a gas at room temp, water is liquid. Stuff that’s a gas at room temp just has less bonding forces (and often mass) than liquids or solids. So they don’t take as much heat to go fast. There’s a lot of heat even at room temp, and even at -40deg. The temperature for nitrogen to sit in one place is -210C or -346F.
Molecules interact with each other. Energy is transferred as they bump around. If you were to follow a single molecule it would move around randomly. What we can measure is usually the average of many molecules.
So it’s less of vibrating and more about smashing around into things?
This is easier to envision with a gas: like a chamber of balks all ricocheting like mad. It’s harder to envision for a solid. But I guess a molecule will be up smack against its neighbors, getting repelled, not so much bounding freely?
Even solids are mostly nothing. This is why neutron stars are so dense - there is a lot less nothing between the neutrons, largely due to gravity.
Here’s another way to think about it. A gas is like a bunch of balls bouncing around a room, hitting the walls and occasionally each other. A solid is like a ball pit, but the balls are vibrating. There is still a lot of bouncing, but most of themstay together.
So in a solid, you can imagine each atom connected to each other by springs (bonds). They can vibrate on these springs. If they vibrate too much (by heating) then they can break the bonds and escape as a gas. Gasses basically have too much energy to bond again.
deleted by creator
This concept isn’t new either. Factories have been using very similar methods to use the heat of the exhaust gasses to power the sensors and whatnot on top of their smoke stacks for some time now, for example.
The article opens with saying only 25% of the fuel’s energy gets used by the motor, 75% is in the heat of the exhaust. I’ll take that as a given. Let’s assume a small motor (in this inventions favour) with a nominal power of only 60 kW, running only at half tilt, 30 kW.
That gives us 90 kW in the exhaust heat by the numbers of the article. So the 56 W it captured in the simulation would be 0.046% of the total 120 kW power being converted by burning the fuel, raising the efficiency from 25% to 25.046%.
The headline is so massively overstated it’s basically just a lie. If the device was built, not just simulated, and you’d manage to substitute part of the alternator’s ouput with the thermoelectic generator’s output, the effect on fuel economy would be below the measurable level.
In simulations mimicking high-speed environments, the waste-heat system demonstrated great versatility; their system produced up to 56 W for car-like exhaust speeds and 146 W for helicopter-like exhaust speeds, or the equivalent of five and 12 lithium-ion 18650 batteries, respectively.
A great step!
Next is build one.
Then cheap-ish and durable.
I doubt this will ever become any useful at all. 50 Watts is nothing compared to what the car engine outputs at high speed. Even a small engine has some 35kW, i.e. 35000 Watts.
I could see it maybe being used as an emergency back up alternator, just enough to keep lights on and engine firing (with storage) to get you to safety.
Do alternators fail that often?
Nope. It does suck when they do.
Wouldn’t be powerful enough actually. You’re better off having a battery that’s in good condition. I’ve done nearly 100 km with the alternator belt off. It being a diesel, it didn’t need to fire sparks though.
It being a diesel, it didn’t need to fire sparks though.
That’s cheating :P
I doubt regenerative braking will be ever useful at all because Lord knows you’re not going to get the same energy back that you took out while driving. /s
This is just to increase efficiency.
Sometimes efficiency gains aren’t worth the cost and complexity. Regenerative braking produces a shitton more power than this, so it’s worth it (also, the motors are already there, just run them in reverse and turn them into generators). You can get the same thing by slapping a solar panel on the roof. Which nobody is doing because it’s too costly and complex for what you get out of it.
Show this to any automotive engineer and count how many seconds before they start laughing
isn’t this basically the old F1 MGU-H system that they kept having issues with and eventually gave up on?
Edit: no, that system, confusingly, is an electric motor/generator connected to the turbocharger.
Was also terribly expensive. The performance to cost wasn’t worth it even for F1
Only getting rid of them next year I’m pretty sure. And initial problems of reliability but not these days. With phenomenal thermal efficiency from tiny engines
OMG they’ve actually introduced the 2025 Turboencapulator.
This type of technology and the concepts of energy conservation and recovery are so damn cool. Think of drainage recovery systems for showers sinks and gutters, sewage, and on and on for various applications and types of recovery.
I remember when I first learned about regenerative braking when the first Priuses were coming out. Shit blew my mind.
Would be cool if Laird or one of the other thermoelectric ceramic (TEC) manufacturers made radial, cylindrical TECs. So far I’ve only seen them be flat and square, or they cut the flat square out like this
instead of in the radial direction. There are some made for research but not commercially available as far as I’ve been able to find.

Also TECs are very brittle too so vibration if that tail pipe would have to be dealt with for them not to crack. I’ve cracked a few myself but i use for heating/cooling in machine design for work.







